KardiaMobile 6L can be used to measure QT duration in COVID-19 patients - Cardiac Rhythm News
Por un escritor de hombre misterioso
5 (622) En stock

KardiaMobile 6L, which it describes in a press release as the world’s only six lead personal ECG.
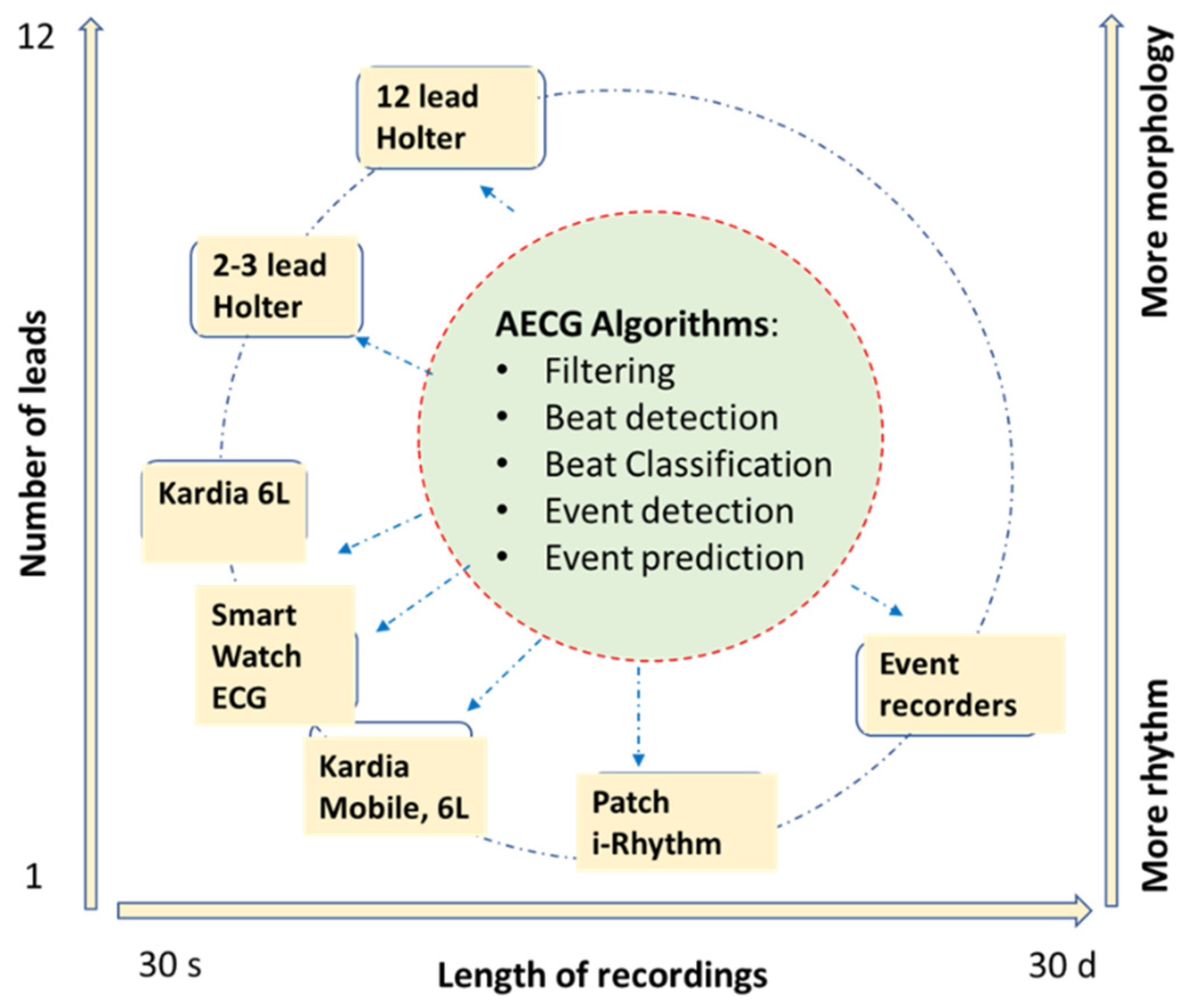
Hearts, Free Full-Text
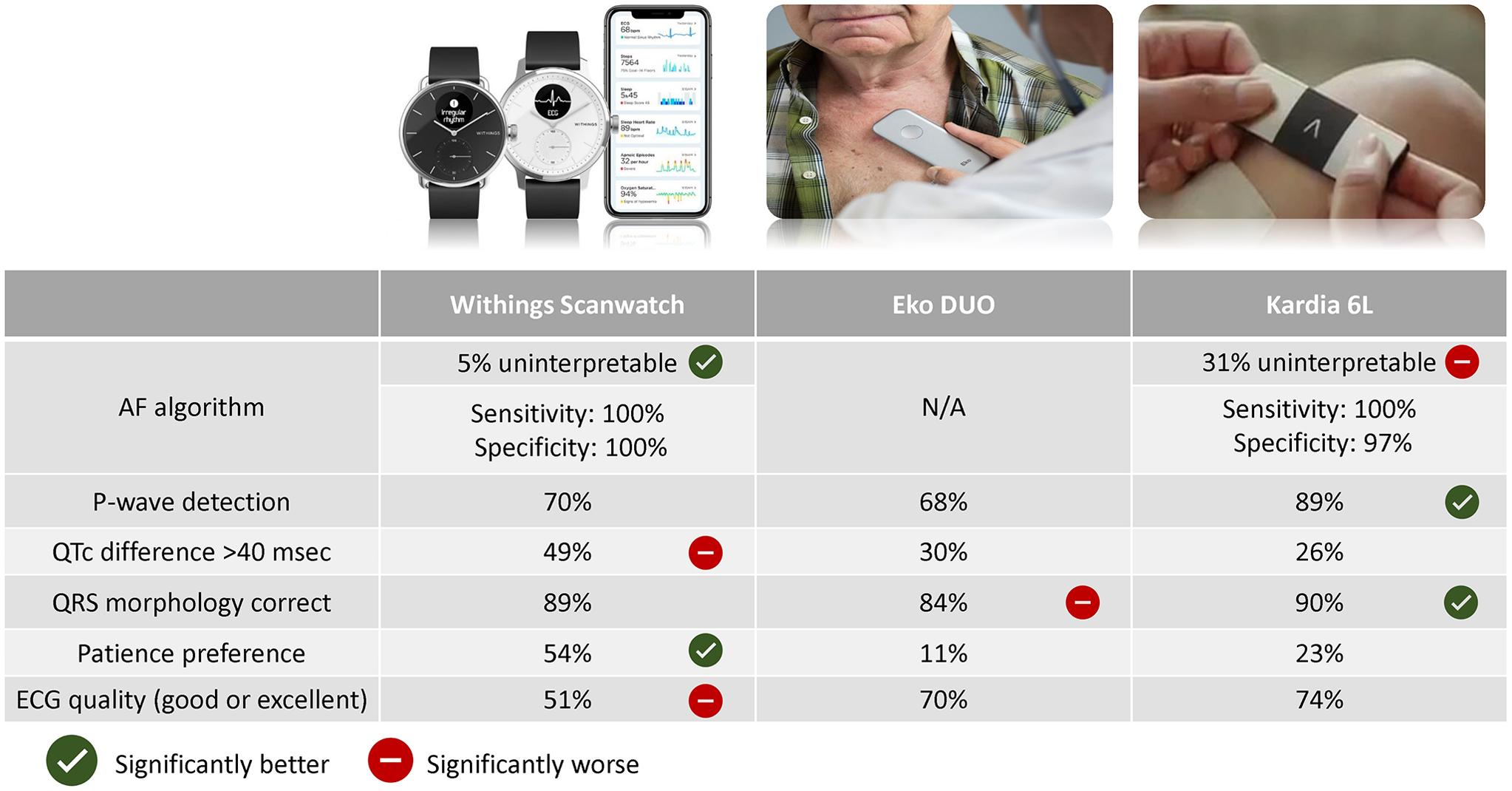
A comparison of ECG-based home monitoring devices in adults with CHD, Cardiology in the Young

Sensors, Free Full-Text
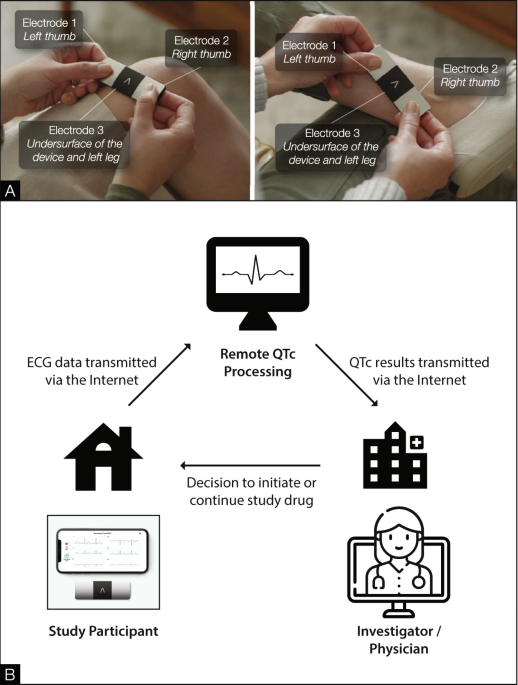
Implementation of a fully remote randomized clinical trial with cardiac monitoring

AliveCor gains US FDA clearance for KardiaMobile 6L to calculate patients' QTc interval - Medical Device News by Guided Solutions

RApid Throughput Screening for Asymptomatic COVID-19 Infection With an Electrocardiogram: A Prospective Observational Study - Mayo Clinic Proceedings: Digital Health

New FDA Guidance Allows Use of KardiaMobile 6L to Measure QTc in COVID-19 Patients

Artificial Intelligence–Enabled Assessment of the Heart Rate Corrected QT Interval Using a Mobile Electrocardiogram Device

Cardio‐oncology care in the era of the coronavirus disease 2019 (COVID‐19) pandemic: An International Cardio‐Oncology Society (ICOS) statement - Lenihan - 2020 - CA: A Cancer Journal for Clinicians - Wiley Online Library

2021 ISHNE/ HRS/ EHRA/ APHRS collaborative statement on mHealth in Arrhythmia Management: Digital Medical Tools for Heart Rhythm Professionals - Varma - 2021 - Annals of Noninvasive Electrocardiology - Wiley Online Library

Using 12-Lead ECG to Detect Drug-Induced Long QT Interval: The Gold Standard
AliveCor Kardia Mobile 6L FDA Cleared- Mobile ECG Device, New
 MAGNUS SPEAKER MKII - para Altavoces Cable de audio de alta gama para altavoces acústicos de alta fidelidad con reducción de ruido
MAGNUS SPEAKER MKII - para Altavoces Cable de audio de alta gama para altavoces acústicos de alta fidelidad con reducción de ruido Cinta Métrica Digital 3 En 1, Herramienta De Medición Láser
Cinta Métrica Digital 3 En 1, Herramienta De Medición Láser Accesorios Xiaomi 11 Lite 5G NE
Accesorios Xiaomi 11 Lite 5G NE Disfraces de pareja Vikinga para adultos: Disfraces parejas,y disfraces originales baratos - Vegaoo
Disfraces de pareja Vikinga para adultos: Disfraces parejas,y disfraces originales baratos - Vegaoo Tetera marroquí hecha a mano importada con filtro integrado, trae a casa una tradición bellamente funcional del Cercano Oriente, 42 onzas (1.25 L) : Hogar y Cocina
Tetera marroquí hecha a mano importada con filtro integrado, trae a casa una tradición bellamente funcional del Cercano Oriente, 42 onzas (1.25 L) : Hogar y Cocina 5 Mejores Microondas con Apertura Derecha del 2024 【MEJOR PRECIO】
5 Mejores Microondas con Apertura Derecha del 2024 【MEJOR PRECIO】